Headline News
Gadolinium Retention In The Human Body: Safety In Children
September 16, 2021
OPINION
GADOLINIUM RETENTION IN THE HUMAN BODY: SAFETY IN CHILDREN
Gadolinium-based contrast agents (GBCA) have been used to enhance MR exams for over three decades. First approved by the FDA in 1988 for clinical use in neuroimaging, they were quickly adopted for imaging of all body parts. GBCAs have exceptional paramagnetic properties, shortening the longitudinal T1 relaxion time and thereby increasing signal on T1-weighted images. GBCAs have excellent safety profile with relatively rare allergic reactions.
 In 2006, an association between GBCAs and nephrogenic systemic fibrosis (NSF) was recognized in patients with renal insufficiency (1). NSF is a widespread progressive tissue fibrosis that predominantly involves the skin, though may affect the lungs, liver, heart, and muscles. While the exact mechanism for this association remains unknown, it was largely associated with nonionic, linear GBCAs. With the move towards utilizing more stable ionic, linear and macrocyclic GBCAs, as well as optimized practice guidelines, no new cases of NSF have occurred since 2009.
In 2006, an association between GBCAs and nephrogenic systemic fibrosis (NSF) was recognized in patients with renal insufficiency (1). NSF is a widespread progressive tissue fibrosis that predominantly involves the skin, though may affect the lungs, liver, heart, and muscles. While the exact mechanism for this association remains unknown, it was largely associated with nonionic, linear GBCAs. With the move towards utilizing more stable ionic, linear and macrocyclic GBCAs, as well as optimized practice guidelines, no new cases of NSF have occurred since 2009.
In 2014, GBCA retention in the brain was inferred in patients with normal renal function (2-4), due to the recognition of hyperintense dentate nuclei and globi pallidi on T1W images. These findings were subsequently validated by several studies (5,6), confirmed to occur in both linear and macrocylic GBCAs (7), as well as confirmed pathologically to not just be isolated to the dentate nuclei and globi pallidi – but occurring elsewhere in the brain (7). Retention of GBCA is not limited to the brain and can occur elsewhere in the human body, such as in bones (8). Gadolinium retention appears to be lower with macrocyclic agents, presumably due to more efficient clearance (9). The mechanism behind and clinical relevance of gadolinium retention is unknown and thus remains controversial. To date, no adverse clinical outcome has been conclusively linked to GBCA retention in the brain.
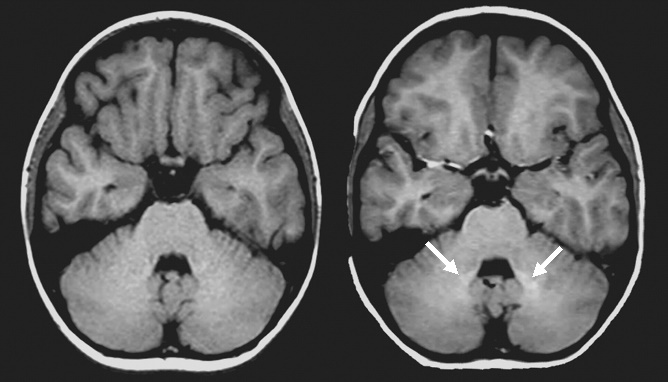
As a result of these findings, the FDA has issued several warnings and updates about GBCAs over the past few years. This included a new class warning requirement and other safety measures for all GBCAs and a patient medication guide to be given to patients prior to GBCA administration (10). They ultimately concluded that gadolinium retention has not been directly linked to adverse outcomes in patients with normal renal function and that the benefit of these agents outweighs any potential risks.
In conclusion, GBCAs accumulate in the human body and brain in trace amounts and with no known definitive clinical consequences. Active involvement of radiologists in protocolling contrast enhanced MRI requests and consultation with the ordering provider may help eliminate unnecessary use of GBCA (11). This may be of particular importance in children with the increased potential to exposure to GBCA over their expected longer lifetime.

Mehmet Emin Adin, MD
Yale School of Medicine
References:
1. Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, Thomsen HS. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. Journal of the American Society of Nephrology. 2006 Sep 1;17(9):2359-62.
2. Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014 Mar;270(3):834-41.
3. Adin ME, Yousem DM, Kleinberg L. Hyperintense dentate nuclei on T1 weighted MRI. XXth Symposium Neuroradiologicum; Istanbul, 2014. O152.
4. Adin ME, Kleinberg L, Vaidya D, Zan E, Mirbagheri S, Yousem DM. Hyperintense dentate nuclei on T1-weighted MRI: relation to repeat gadolinium administration. American Journal of Neuroradiology. 2015 Oct 1;36(10):1859-65.
5. McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015 Jun;275(3):772-82.
6. Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Investigative radiology. 2014 Oct 1;49(10):685-90.
6- Kanda T, Osawa M, Oba H, Toyoda K, Kotoku JI, Haruyama T, Takeshita K, Furui S. High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology. 2015 Jun;275(3):803-9.
7- Murata N, Gonzalez-Cuyar LF, Murata K, Fligner C, Dills R, Hippe D, Maravilla KR. Macrocyclic and other non–group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Investigative radiology. 2016 Jul 1;51(7):447-53.
8. Lord ML, Chettle DR, Gräfe JL, Noseworthy MD, McNeill FE. Observed deposition of gadolinium in bone using a new noninvasive in vivo biomedical device: results of a small pilot feasibility study. Radiology. 2018 Apr;287(1):96-103.
9. Jost G, Frenzel T, Boyken J, Lohrke J, Nischwitz V, Pietsch H. Long-term excretion of gadolinium-based contrast agents: linear versus macrocyclic agents in an experimental rat model. Radiology. 2019;290(2):340-8.
10- United States Food and Drug Administration (2018) FDA drug safety communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings. FDA document. https://www.fda.gov/drugs/drug-safety-andavailability/fda-drug-safety-communication-fda-warnsgadolinium-based-contrast-agents-gbcas-are-retained-body. Accessed August 22nd, 2021
11. Adin ME, Yousem DM. Gadolinium retention in humans: survey of radiologists and impact on daily practice. J Ist Faculty Med. Published online May 27, 2021. doi: 10.26650/IUITFD.2021.874672